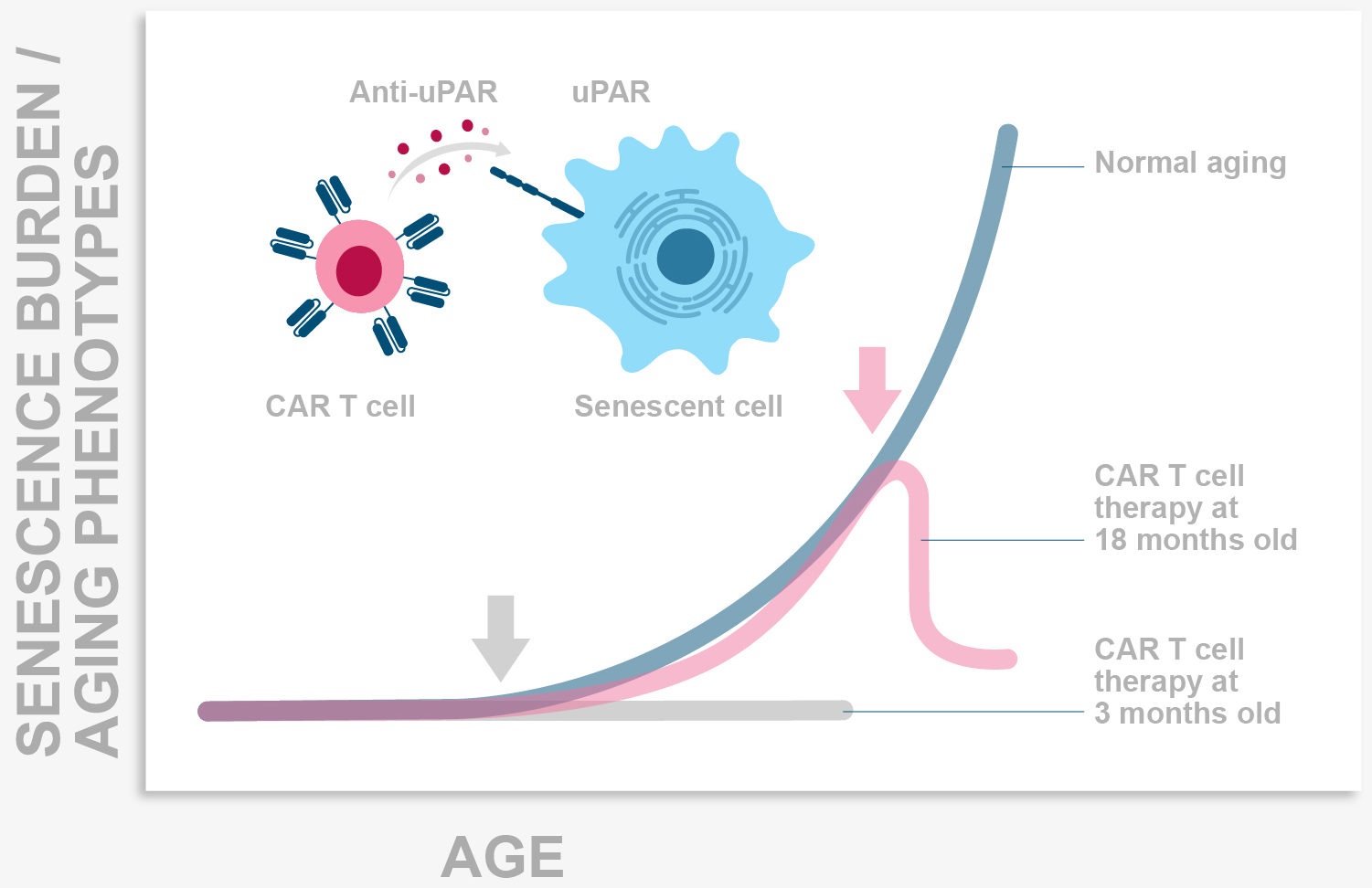
Senescence is a stress response program whereby damaged cells stop replicating and performing their functions and instead become highly proinflammatory. In physiological conditions senescent cells are eliminated by the immune system.
During aging however, the increase in damaged cells plus the decreased efficiency of the immune system leads to the accumulation of senescent cells in tissues where they generate a chronic proinflammatory milieu that drives age pathologies. In our lab we seek to understand how the immune system targets these cells and to develop immune-based senolytics as therapeutic approaches for age-related diseases including cancer.